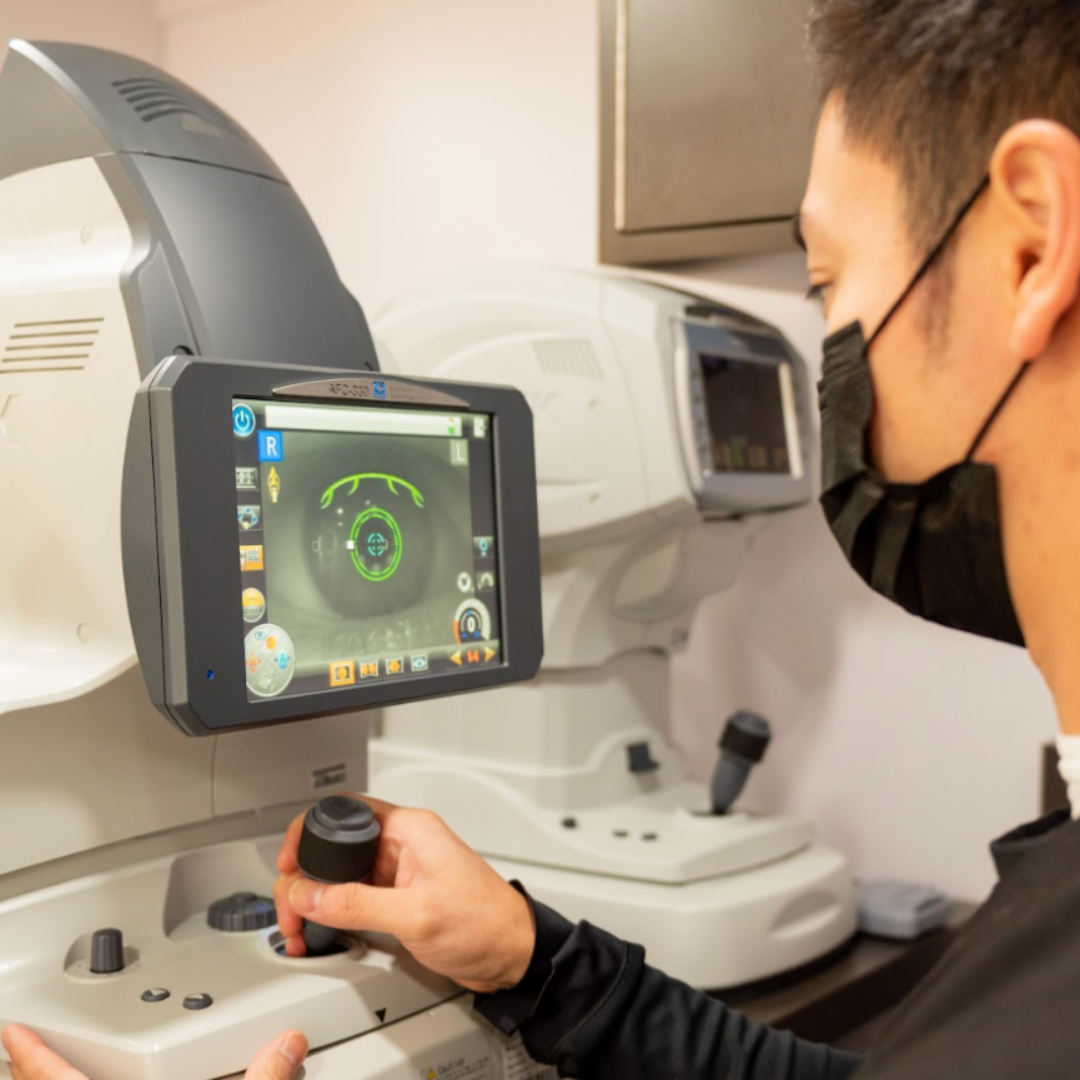
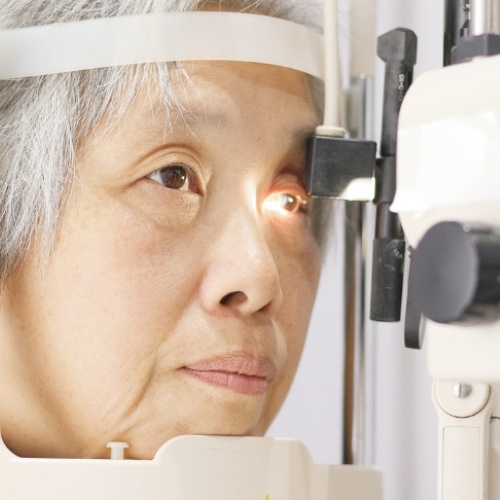
Clinical Trial Solutions
Ensure compliant trial designs, maintain budgets, and hit timelines with the only provider dedicated to point-of-need eye exams for clinical trials.
With over 80% of US clinical trials failing to meet their patient enrollment timelines and an average of 30% of patients dropping out, point-of-need ophthalmic clinics are a game changer.

At 20/20 Onsite, we specialize in delivering comprehensive mobile ophthalmic solutions that streamline every phase of clinical trials. Our mobile eye services integrate seamlessly into your study—from thorough protocol reviews and precise prescreening to optimizing patient enrollment and retention strategies—ensuring superior study outcomes. By efficiently integrating assessments, we provide a holistic approach to clinical trials that meets the rigorous standards of clinical research.
Point of Need
100%
Screening timelines met across all business operations.
Data Integrity
98%
Data accuracy submission rate.
Patient Retention
91%
Patient retention rate vs industry standard 70%.
Patient Centricity
95+
Patient NPS score.
Point-of-need Clinical Trial Solutions
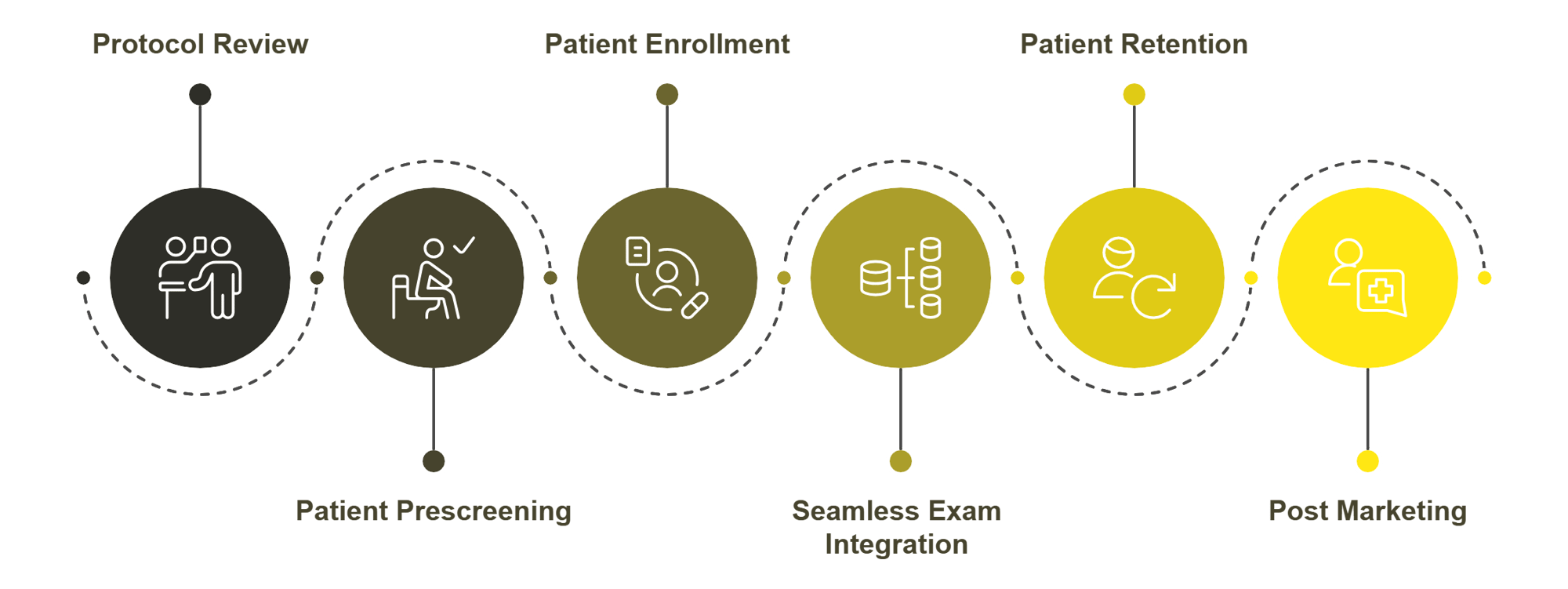
Study Design and Protocol Review

We ensure that every aspect of your study is aligned with its ocular endpoints, resulting in compliant trial designs that meet critical timelines and schedules. With a proven track record of refining protocols for numerous multi-center studies, we consistently improve clinical trial outcomes.
- Expertly aligning ocular assessments with study endpoints to ensure robust, compliant trial designs and maintain budgets, timelines, and schedules.
- Tailoring patient recruitment and protocol strategies to enhance efficiency and safeguard trial success.
Our prescreening process harnesses advanced analytics and targeted outreach to identify the candidates for your clinical study. By thoroughly evaluating patient profiles early on, we significantly reduce screening times and ensure that only the best-fit participants move forward.
- Utilizes data-driven insights to target and identify ideal candidates precisely.
- Streamlines initial outreach and evaluation, reducing screening time and enhancing candidate suitability.
Patient Prescreening
Patient Enrollment

Our patient enrollment strategy is centered on creating a smooth and engaging enrollment process that minimizes barriers to entry. We combine point-of-need assessments with personalized support to efficiently enroll patients while strictly adhering to regulatory standards.
- Implements a user-friendly, patient-centric exam assessment schedule to improve patient engagement.
- Ensures swift, compliant onboarding that accelerates the start of your clinical trial.
We integrate directly into your clinical trial workflow, ensuring that each ophthalmic assessment perfectly aligns with study requirements and does not disrupt your current site workflow. This integration reduces disruptions and maintains the integrity of critical data throughout the trial.
- Synchronizes point-of-need vision clinics with established clinical workflows for seamless execution.
- Optimizes exam workflows to ensure continuous, high-quality data collection and minimal process disruption.
Seamless Exam Integration

Patient Retention

Our retention strategy recognizes that ongoing patient engagement is vital for trial success and emphasizes continuous communication and point-of-need support. We proactively keep participants informed, engaged, and motivated throughout the study.
- Employs proactive communication, scheduling, and follow-up strategies to sustain engagement.
- Uses educational tools and support to foster a positive trial experience.
Ensure that trial patients continue to receive the follow-up assessments required by your study, wherever they are. Our point-of-need assessments help analyze long-term outcomes to keep patient safety at the forefront.
- Conducts comprehensive point-of-need assessments to evaluate real-world effectiveness and long-term outcomes.
- Keep patient safety and compliance at the forefront with our point-of-need, flexible post-market solutions.
Post Marketing
Take a Tour
Tour our patient-centric, point-of-need Mobile Vision Clinic (MVC) and see how we accelerate clinical trials.
Clinical Trials Phase 1-3
Support for ophthalmic assessments or other study-specific eye-related solutions as part of a research protocol.
Post Marketing
Support for safety or surveillance ophthalmic assessments and eye-related clinical trial solutions for patients outside of a clinical protocol.

Mobile Vision Clinic
.png)
Mobile Vision Suite
-1.png)
Mobile Vision Pod

Applied Genetics Technology Company (AGTC) partners with 20/20 for their nationwide ophthalmic phase 1 and 2 clinical trials
Meet 20/20 Onsite's Industry and Scientific Advisory Board
The Industry and Scientific Advisory Board (IASAB) meets quarterly to help identify patient needs, product opportunities, and market challenges associated with the provision of vision-related assessments for clinical trials and safety surveillance.

Rajat Agrawal, MD, MS
CEO and President Retina Global

Jeanne Hecht, Chair
Founder and CEO JTH Consulting, LLC

Jeffrey S. Heier, MD
Director of the Vitreoretinal Service and Director of Retina Research Ophthalmic Consultants of Boston (OCB)

Peter K. Kaiser, MD
Chaney Family Endowed Chair in Ophthalmology Research and Professor of Ophthalmology Cleveland Clinic Lerner College of Medicine

Beth Marsh
Vice President of North America Sales and Marketing, Ophthalmology Apellis Pharmaceuticals

Jason Menzo
Chief Operating Officer Foundation Fighting Blindness

Adam Ramsey, OD
Owner Socialite Vision

Click below to read more about each board member's commitment to research or submit a question to be asked during our next quarterly meeting.
Mobile Vision Clinics (MVCs)
.png)
Fully equipped clinics on wheels providing comprehensive ophthalmic assessments essentially anywhere, eliminating geographical barriers.
- MVCs are flexible solutions that eliminate geographical barriers and allow for point-of-need assessments, improving patient access and diversity for clinical trials.
- MVCs have traveled over 200,000 miles across 48 states in the past year.
Mobile Vision Pods (MVPs)

Semi-permanent examination spaces that can be easily set up and customized per specific study requirements. MVPs can be deployed externally at research sites or convenient locations for extended periods.
- MVPs' customizable design allows them to fit in various spaces and be tailored to specific study requirements, offering a cost-effective and adaptable solution.
- MVPs have been successfully deployed in multiple clinical trials, providing a dedicated and comfortable environment for on-site ophthalmic assessments.
Mobile Clinic Suites (MCSs)
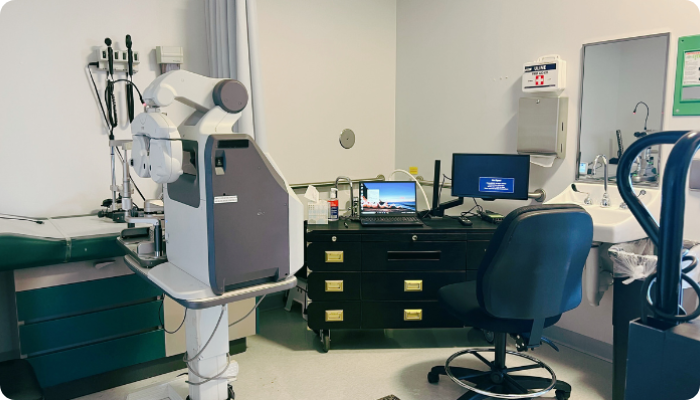
Portable solutions that bring ophthalmic equipment and certified staff to existing exam rooms and suitable facilities.
- MCSs seamlessly integrate with existing clinic workflows and minimize disruption, making them ideal for sites with limited space or that require specific scheduling flexibility.
- MCSs have been utilized to rescue clinical trials facing challenges with ophthalmic assessments. They ensure the timely completion of studies and data collection.
Book a call today to ensure trials stay on track with the only provider dedicated to point-of-need eye assessments for clinical research.
SIGN UP: EYES ON RESEARCH
Our newsletter dedicated to clinical research, insights on patient-centric treatments, and point-of-need clinical trial solutions.
Copyright © 2025. | Privacy Policy